Regenerative medicine holds immense potential to bring new treatments to patients with high unmet medical needs or, indeed, solutions for diseases with no existing treatment options. While it is still a nascent industry, it is evolving at an increasing speed with already close to 1 200 developers of cell therapies including over 650 private entities1 advancing programs and new companies entering the industry each quarter. The increase in research and development initiatives in this field and the successful advancements of programs through the various clinical phases indicates that an industry that will intensify and deliver in the next few years2.
TreeFrog is a regenerative medicine biotech with a unique technology platform set to become a standard in the industry, breaking many of the field’s bottlenecks with internal best-in-class programs in Parkinson’s and acute liver failure, in addition to partnering in other therapeutic areas. As a company with the ambition to make cell therapy available to all, we keep a very close eye on the market and its dynamic evolution. Through a series of articles, we will be sharing some of our findings with you. This first article focuses specifically on the iPSC trials landscape.
Introduction
Stem cells can be grouped into four broad categories based on their origin: embryonic stem cells (ESCs), fetal and adult stem cells, and induced pluripotent stem cells (iPSCs) (Kolios et al. 20123). iPSCs are a type of pluripotent stem cell holding promise in the field of regenerative medicine with unique properties of self-renewal and differentiation to many types of cell lineage (Singh et al. 20154). Since inception in 2018, we use iPSC in our lead programs in Parkinson’s Disease and Acute Liver Failure.
A review from Ilic et al.5 provided an overview of the clinical landscape of pluripotent stem cells in 2021, including trials exploring both ESCs- and iPSCs-derived cell therapies. Focusing specifically on iPSCs, we performed an analysis to assess the evolution of the iPSC trial landscape from 2021 to mid-20246, excluding iPSC-derived immuno-oncology therapeutic approaches, and focusing on regenerative medicine (denomination used to refer to cell therapy outside oncology). We focused on i) identifying new trials and ii) studying their characteristics and iii) reporting on the progress of previously identified clinical trials from 2021. Below are the highlights of our analysis.
20+ new trials exploring iPSC-derived cell therapies emerging since 2021
Since the review from Ilic et al. performed in 2021 in which 35 iPSC trials had been identified, 24 new iPSC trials have emerged. Interestingly, in comparison, while 49 trials exploring ESC-derived had been identified back in 2021, only 15 new trials have emerged since.
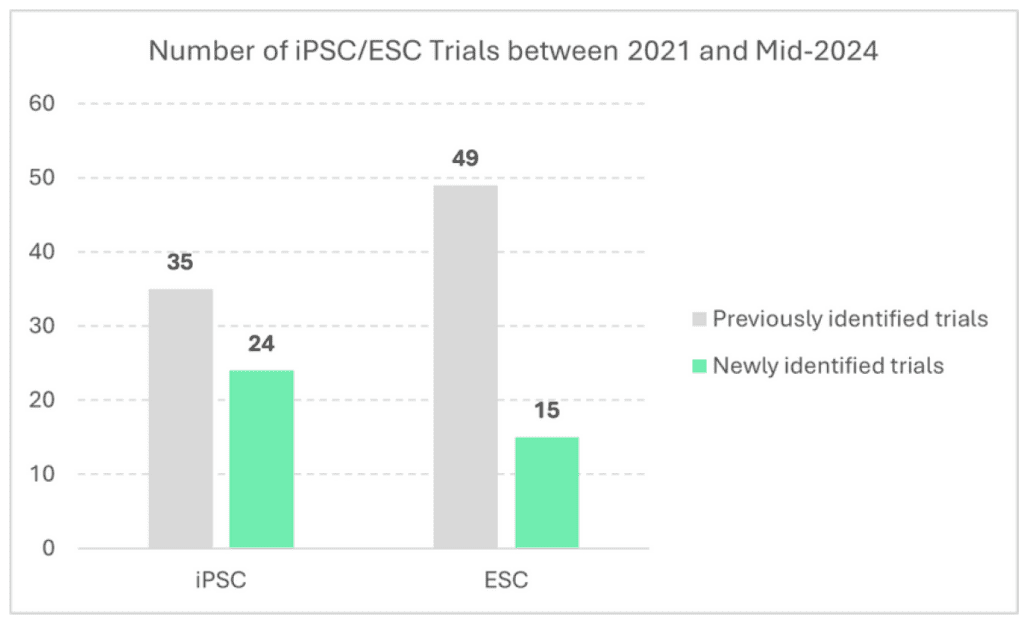
Looking at the characteristics of the newly iPSC identified trials, all are Phase I and II, with similar characteristics as previously identified trials – 92% (n=22) being interventional (as opposed to observational), 88% (n=21) developed by companies (as opposed to academics, 96% (n=23) assessing cell therapies (as. opposed to gene-modified cell therapies) and the majority of trials undertaking investigations in Asia – Pacific region (63%, n=10) with a few in North America (31%, n=5 – note: geography available for only 16 newly identified trials).
New trials exploring iPSC-derived cell therapies are mainly explored for brain, heart, eye and cartilage indications
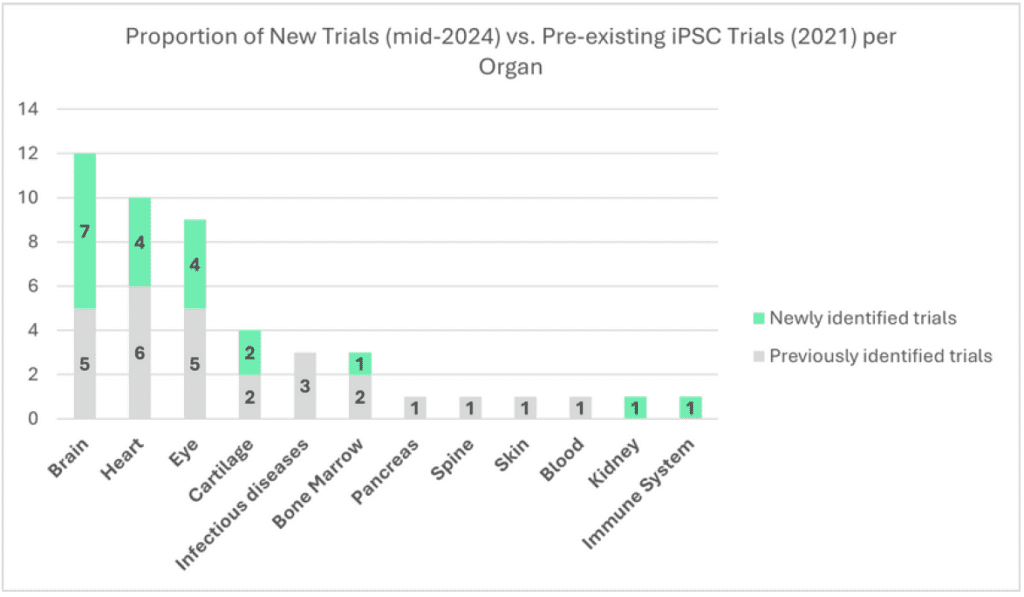
Focusing on interventional trials from companies (i.e. total of 20 out of the 24 new trials), we assessed the organ categories where new investigations occurred.
We identified that new trials have emerged among the brain (+7), heart (+4), eye (+4), cartilage (+2), bone marrow (+1), kidney (+1) and immune system (+1). Interestingly, iPSCs clinical investigations have also been initiated in two new therapeutic fields: Kidney (Kidney Transplant Rejection), and the immune system (Systemic Lupus Erythematosus). During the same period, no new trials emerged in indications associated with infectious disease, pancreas, spine, skin and blood.
+6 new trials explored to address Parkinson’s disease since 2021
When we look further into the evolution at an indication level in most active organ categories, we find, in the brain category, no new therapeutic indication emerged but six new trials in Parkinson’s disease appeared (one other trial started in acute ischemic stroke). For heart diseases, we can see a continuous development in the field of heart failure with four new trials. Meanwhile, in ophthalmology, one new trial appeared in dry age-related macular edema, one in retinitis pigmentosa and two in retinal pigment epithelium impaired diseases. Finally, two new trials emerged in the cartilage organ category. In terms of companies entering the industry, among these most active therapeutic fields, seven players were not present in 2021 (Aspen Neuroscience, HeartWorks, iRegene Therapeutics, Century Therapeutics, Nuwacell Biotechnologies, Eyestem Research and Cline Scientific).
Multiple iPSC-derived cell therapy programs carried over since 2021
As we continued our deep dive, we also looked at the evolution of previously identified programs to assess their progress.
Focusing on interventional trials from companies assessing company assets (i.e. total of 27 out of the 35 previous trials, constituting 24 unique programs), we found that the majority pursued their development (54%), while the rest of the programs had either an unclear status (33%, status assigned to programs with trials no longer ongoing and no clear communication from the company on the future of the program ), or were terminated (13%, status assigned to programs for which the companies have clearly stated the termination of the programs).
Among programs pursued (54%, N=13), the large majority (77%, N=10) were classified as such due to their status (still recruiting) or status evolution (e.g. from recruiting to completed). All of them are Phase I-I/II trials, apart from the CYP-004 program (Cynata Therapeutics) enrolling patients with osteoarthritis in a Phase III trial (ran exclusively in Australia). The three other programs were classified as being pursued as the asset was either explored for another indication (N=1, ALF-202 from Allife Medical now explored for acute ischemic stroke), expanded geographically (N=1, DSP-1083/CT1-DAP001 from Sumitomo Pharma for Parkinson’s disease explored in the US after being investigated in Japan) or evolved to the next phase of development (N=1, CYP-001 from Cynata Therapeutics explored for acute graft-versus-host disease or GvHD transitioned from Phase I to Phase II).
Focusing on the evolution per organ category, two programs in Parkinson’s disease have been continued, while three other programs have an unclear status (Parkinson’s and stroke). In the heart category, all three programs continued (ischemic heart disease, chronic heart failure and congenital heart failure), as the two identified osteoporosis programs in the Cartilage category. Two ophthalmology programs have also continued (bullous keratopathy, retinitis pigmentosa). The status of the rest of the programs is unclear. Other categories have seen their single programs continue (GvHD, spinal cord injury, thrombocytopenia, diabetic foot ulcer), while three programs in infectious diseases (Covid-19, HIV) have been terminated.
Conclusion
The analysis demonstrates the complex nature of early clinical trials with iPSC and the variety of use cases. Overall, the figures support the fact that the field of iPSC research continues to generate traction with many new programs reaching the clinic over the last 3 years. In addition, although some programs have been terminated (13%) or have an unknown status (33%), which could be expected at early phases, the continuation & advancement of a considerable number of programs (54%) demonstrates that iPSC-based cell therapy has a strong potential for further clinical development
Finally, our analysis confirms a strong momentum in Parkinson’s disease research and development.
At TreeFrog, we are currently developing the only product with the presence of mature neurons, in a unique 3D Micro tissue format. Extensive pre-clinical data demonstrates functionality, enhanced cell survival post-transplantation, and good integration of the graft. The proprietary technology production platform is commercial scale up ready with doses for 100s patients per batch. TFG 001 is a potential best in class therapy for Parkinson’s disease and will be ready for first-in-human in 2026.
Further analyses to come
We will be continuing this series of articles with a thorough analysis of the evolution of iPSC clinical trials among selected organ categories. Based on this first analysis, our next article will deep dive into iPSC clinical activities for brain-related pathologies such as Parkinson’s disease.
References
1Alliance for Regenerative Medicine – Investment Sector Data (Q1 2024, analysis as of April 2024)
2 Investing in Stem Cells: Promising Technologies and Market Opportunities, nForming (January 2024)
3 Kolios et al. 2012. Introduction to Stem Cells and Regenerative Medicine. Respiration.
4 Vimal K. Singh. 2015. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol.
5 Dusko Ilic. 2022. Pluripotent Stem Cells in Clinical Setting—New Developments and Overview of Current Status. Stem Cells.
6 Methodology: Update performed using the following resources: GlobalData (paid subscription); clinicaltrial.gov; hPSCreg). Clinical trial data represent a snapshot in time (taken in May 2024)
