Welcome to the second article in our series that presents the findings of our analysis on the market evolution of regenerative medicine in cell therapy.
In our first article (here), we looked at the overall potential of regenerative medicine and the evolution of the induced pluripotent stem cell (iPSC) clinical trials landscape. We demonstrated that despite the challenging financial environment and the complexity of bringing cell therapies to the market, the iPSC clinical trials landscape is a dynamic space with 24 new trials emerging between 2021 and 2024, overtaking new trials using embryonic stem cells (ESC), of which there were 15.
We identified that the most dynamic space is in diseases related to the brain, with six new trials exploring iPSC-derived cell therapies for Parkinson’s disease identified between 2021 and May 2024*. In fact, since the publication of the first article in september 2024, several trials have emerged with the publication of additional protocols and the announcement of expected trials in the upcoming months, further confirming the dynamism of the space.
In this article we will narrow this further down to the evolution and characterization of iPSC- and ESC- cell therapy programs explored in Parkinson’s disease, particularly relevant to TreeFrog Therapeutics, with our lead program in R&D in this field, and as most effort with CNS-targeted therapies involve the treatment of Parkinson’s disease.
High burden and unmet need associated with Parkinson’s disease
Parkinson’s disease is the second most common neurodegenerative disorder after Alzheimer’s disease. It is a progressive disease that is mainly characterized by loss of dopaminergic neurons in the substantia nigra. Symptoms usually occur when 60-80% of these neurons are already lost, so diagnosis is usually quite late in terms of disease progression. The symptomatology of Parkinson’s disease is acknowledged as heterogeneous, consisting of a mix of motor symptoms (e.g. bradykinesia, rigidity, resting tremor) and non-motor symptoms (cognitive deficits, autonomic dysfunctions and mood disorders)1. Pharmacological treatment initially provides significant relief from motor symptoms, often referred to as the “honeymoon” phase, lasting from 3 to 6 years. However, as the disease progresses, DA neurons continue to degenerate and the effectiveness of available treatment options gradually declines, leading to motor fluctuations and complications such as wearing off effects and dyskinesias affecting 60% of patients2.The last resort for patients is Deep Brain Stimulation (DBS), which, like pharmacological treatments, only provides symptomatic relief without restoring the degenerated dopaminergic neurons and carries the risk of diminishing motor benefits over time. This highlights the need for new therapies that regenerate the lost DA neurons. There is a huge unmet need with a prevalence that has doubled in the past 25 years, to a global estimate of 10million patients3. Disability and death are increasing faster than any other neurological disorder and the annual cost burden is set to increase significantly – It currently stands at $52billion in USA and $15billion in Europe 4.
Stem cell-derived cell replacement, a rational option to address Parkinson’s disease
Research into cell replacement strategies for Parkinson’s disease has been ongoing for decades. In fact, the first clinical trial of a cell-based therapy for humans with Parkinson’s disease was conducted in the late 1980s 5.
Given the well-characterized pathophysiology of Parkinson’s disease—marked by the localized degeneration of nigral dopaminergic neurons—a restorative strategy aiming to replace the lost DA neurons has emerged as a particularly relevant approach to address motor symptoms. Intensive research efforts are now being translated into clinical applications, shaping a rapidly evolving clinical landscape.
We studied the evolution of trials exploring cell transplantation (with a focus on ESC and iPSC-derived therapies) between 2021 and May 2024, and further re-assessed the landscape in Q1 2025. The analysis is restricted to interventional trials exploring derived company-licensed assets (exclusion of observational trials and trials exploring assets not identified as company-licensed).
A dynamic field of research translating into active clinical investigations
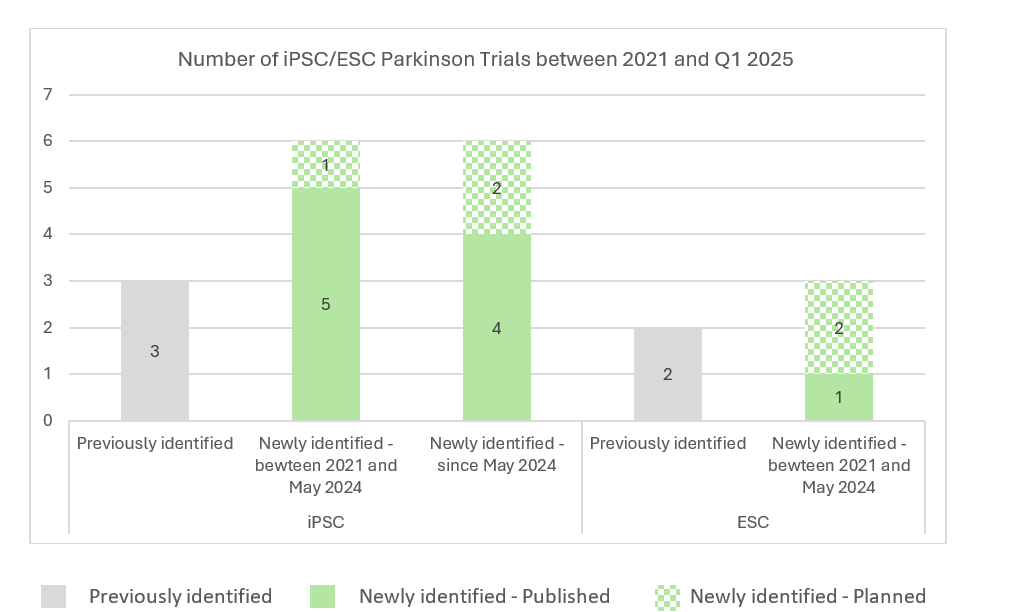
As shown on the graph below, multiple new trials exploring iPSC-/ESC- derived cell therapies have emerged since 2021, showing high interest in developing a cell therapy strategy to address such indication. Aligned with the analysis performed on all iPSC-/ESC-derived trials, the proportion of new trials assessing ESC-derived cell products is less as compared to new trials assessing iPSC-derived cell therapies, further demonstrating the increased potential of iPSCs in cell therapy.

Back in 2021, five trials/programs iPSC-/ESC- derived cell therapy approaches in Parkinson’s disease had been identified. Among these five trials/programs, two appear no longer active (program from Allife Medical Science – NCT03815071; program from Technology and International Stem Cell/Cyto Therapeutics – NCT02452723) as no subsequent investigations from both players have been identified. On another hand, three programs have been pursued, among which DSP-1083/CT1-DAP001 from Sumitomo Pharma, whose clinical studies have been expanded geographically from Japan (UMIN000033564) to the U.S. (NCT06482268, NCT06753331), bemdaneprocel from BlueRock Therapeutics which recently completed a Phase I study (NCT04802733) and NN-9001 from Novo Nordisk/Lund University currently conducting a Phase I study (NCT05635409).
New trials have been initiated by players already running clinical investigations with their product back in 2021, but several new players have also progressed to clinic such as Aspen Neuroscience (NCT06344026), iRegene Therapeutics (NCT06167681), XellSmart Biomedical (NCT06145711) and S. Biomedics (NCT05887466), all currently running Phase I – I/II studies.
Among newly identified trials since May 2024, iRegene Therapeutics recently initiated a new trial exploring NouvNeu001 for early-onset Parkinson’s disease (NCT06608355). We have also recently identified a trial sponsored by Penelope J. Hallett in collaboration with Oryon Cell Therapies (NCT06422208). Two additional players, Shanghai Yuesai Biotechnology and iCamuno Biotherapeutics, recently emerged with the recent publication of two Phase I trials (ChiCTR2500095374/NCT06778265; NCT06821529).
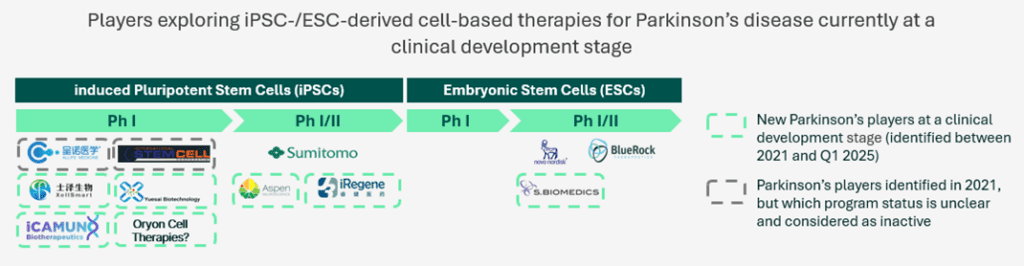
Other competitors currently in preclinical studies are also expected to initiate clinical studies from 2025, such as Kenai Therapeutics developing an iPSC-derived cell-based therapy, while well-established players are expected to pursue clinical investigations with new trials to initiate in the upcoming months. Our cell therapy for Parkinson’s disease, TFG 001, is currently in preclinical investigations, and on track to be ready for first-in-human in 2027.
Interestingly, most active programs are currently being conducted either in the North America or Asia-Pacific regions with a single program conducted by Novo Nordisk/Lund University in EU. This not only illustrates the complexity of bringing cell therapies in different regions (differing guidelines, regulatory requirements etc.) but also means that, at least initially, only a portion of the patient population (mainly US and Japan) may have access to the first cell therapy treatments for Parkinson’s disease.
Cell therapy approaches explored in clinical investigations
Cell product: As Parkinson’s disease is caused by the degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNpc)6, replacement of the primary dysfunctional cell population in the disease, i.e. dopaminergic neurons, has been the major focus of cell replacement therapies being developed over the past years. Assessing the cell type product of cell therapies from active programs, we identified that the large majority of the programs in active clinical development explore cell therapies producing either dopaminergic neural precursors or progenitors.
Format: The majority of active clinical programs explore cell-based products consisting of suspensions of cells i.e. midbrain neural precursors or progenitors. The challenge of using single-cell suspension is the risk of inducing programmed cell death through anoïkis7, affecting the survival and/or the potency of the product post-transplantation, hence the control of the final dose (active dopamine neurons).
Transplant type: There are two main types of stem cell transplants, those derived from a patient’s own cells (autologous) and those derived from a donor’s cells (allogeneic). About half of the identified active programs in clinic explore an allogeneic approach, the other half exploring an autologous approach. There are some advantages of the autologous approach as using a patient’s own cells reduces risk of rejection graft versus host disease (GvHD) and patients do not need to receive immune-suppression treatment. However, an autologous approach is personalized as using patients’ sampling, which can prompt logistics challenges and increased production expenses as for each patient you will need to generate a pluripotent cell line. Therefore, autologous treatments, while offering a tailored approach, face considerable practical hurdles. Allogeneic therapies offer scalability and the potential for “off-the-shelf” products. This approach benefits from standardized production and reduced costs, but faces challenges related to immune rejection. 8
Results: Positive safety data with cell-based therapy studies in Parkinson’s disease recently confirmed by ongoing trials’ results
Overall, the field is advancing with promising clinical data emerging from programs such as BlueRock Therapeutics which showed continuous favorable safety profile at 24 months in all 12 patients enrolled in the Phase I exPDite trial (NCT04802733) 9. S.Biomedics also shared positive one year safety results from 12 patients enrolled in the ongoing Phase I/II study (NCT05887466), showing no adverse effects related to the cell transplantation or surgery 10. Data from the investigator-initiated study conducted by Kyoto University (UMIN000033564) have been released in 2024. Based on these data, the company prepares for NDA submission to secure approval in this geography11.
TreeFrog’s differential cell therapy approach to Parkinson’s Disease
At TreeFrog Therapeutics, our approach to cell therapy development is unique and based on a transformational technology that we have developed in-house, C-Stem™, that can amplify and differentiate cells in one closed system.
In contrast to the other cell therapy approaches mentioned in this article, TreeFrog’s program has several differentiating factors with excellent results that overcome some of the challenges that still exist with therapies further advanced in clinic.
Cell Type: The cell therapies currently in clinic are the majority on precursors and progenitors – i.e. the cells giving rise to midbrain cells including DA neurons. Our cell therapy contains dopaminergic neurons (in addition to progenitors) and has demonstrated a fast time to effect in pre-clinical models. Results have shown full recovery within 16 weeks, sustained at eight months12.
Format: Classic cell therapies primarily use single cells in suspension which have an elevated risk of cell death13. With our C-Stem™ technology, we produce 3D microtissues that have unique benefits. The dopaminergic neurons and progenitors in the microtissue are spatially organized in a cohesive network; a microenvironment that provides protection to the cells and facilitates their transplantation. Results have shown they integrate well in the brain.”
Scale: One of the major challenges facing all cell therapy developers is scale. At present, companies are focused primarily on a scale-out model which is manually intensive and difficult to monitor in terms of quality control. Our GMP-compliant technology provides end-to-end automated scale-up and we are currently manufacturing at commercial scale.
Cryopreservation: We demonstrated the functionality of cryopreserved neural microtissues12, enabling the practical implementation of a scalable process.
The next article will explore the unmet needs in liver disease, the current advancements in cell therapy, and how these approaches contrast with Parkinson’s disease in terms of scale, complexity, and clinical application.
